
How to Start a Pharmaceutical Company in India: A Comprehensive Guide 2024
Don’t know how to start a pharmaceutical company in India? The Indian pharmaceutical sector has very high growth potential, being third in the world in terms of volume. This comprehensive guide is prepared for new entrepreneurs who want to embark on the difficult yet rewarding journey of establishing a pharmaceutical company in India.
Understanding the Indian Pharmaceutical Landscape
Understanding the market is the first step when learning how to start a pharmaceutical company in India. There is an expectation that India’s pharmaceutical industry will grow to $130 billion by 2030, which is favorable for the entrepreneurs. Being the ‘pharmacy of the world’ also affords the country many advantages with new entrants.
Legal Structure and Initial Registration
Selecting the right business structure is very important when trying to figure out how to start a pharmaceutical business in India. Here is a detailed explanation below:
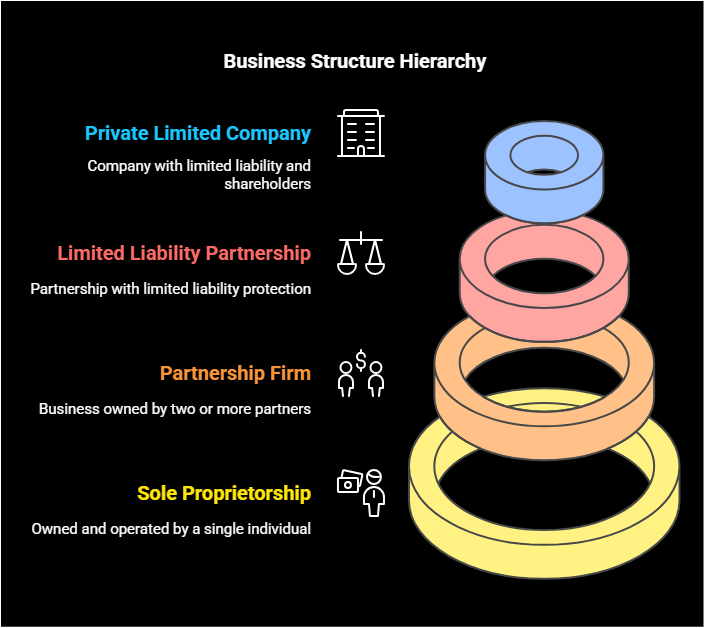
Business Structure Options
- Private Limited Company (Most Recommended) : – Company with limited liability and shareholders
- Limited Liability Partnership (LLP) :- Partnership with limited liability protection
- Partnership Firm : – Business owned by two or more partners
- Sole Proprietorship :- Owned and operated by a single individual
Essential Registration Steps
Understanding the concepts of registration and incorporation of companies in India, it may be necessary to first familiarise oneself with a set of Indian terminologies below. These terms and their definitions will help ease understanding of the document provided.
- Director Identification Number (DIN) – An identification number meant for an Individual intending to be a director in a company in India.
- Certificate of Incorporation – A certified document confirming the incorporation and existence of a company under the Companies Act.
- GST Registration – Registration that enables a business entity, having taxable supplies, to collect and pay Goods and Services Tax (GST).
- Professional Tax Registration – A registration for tax at the state level where a business entity is expected to withhold and remit a professional tax on employees.
- ESI & PF Registration – Registration with the Employee State Insurance Corporation for Employees State Insurance (ESI) and Provident Fund (PF) to provide social security to employees.
- Import-Export Code (IEC) – A code issued to a person who intends to engage in the business of import or export of goods and services.
Detailed Licensing Requirements
A critical aspect of how to start a pharmaceutical company in India involves obtaining necessary licenses. Here’s what you need:
1.Manufacturing Licenses
- Drug Manufacturing License: Issued by the State Drug Control Authority, it is a license to manufacture drugs.
- Schedule M Compliance Certificate: This certificate is issued under Schedule M of the Drugs and Cosmetics Act, a certificate of good manufacturing practices (GMP) compliance.
- State Drug Control Authority Approval: The approval to function based on the laws of the specific state drug control authority.
- FDA Certification: Certification that is issued ensures that the medicine is made under all the specifications maintained by the Food and Drug Administration.
- WHO-GMP Certification: This certification is necessary for the export of drug products which indicates that international quality norms are followed.
2. Environmental Clearances
- Pollution Control Board Approval: Clearance from P C B for the construction of the pharmaceutical unit regarding pollution control clearance.
- Environmental Impact Assessment (EIA): Study to understand and reduce the effect of the manufacturing unit on the environment.
- Hazardous Material Handling Permits: Approval for the management of hazardous material by safe, storing and disposing methods.
- Waste Management Certification: A certificate issued for ensuring waste disposal is done according to environmental standards.
Infrastructure Development
Successfully learning how to start a pharmaceutical company in India requires careful infrastructure planning:
Manufacturing Facility Requirements
- Minimum 1500 square feet for basic operations
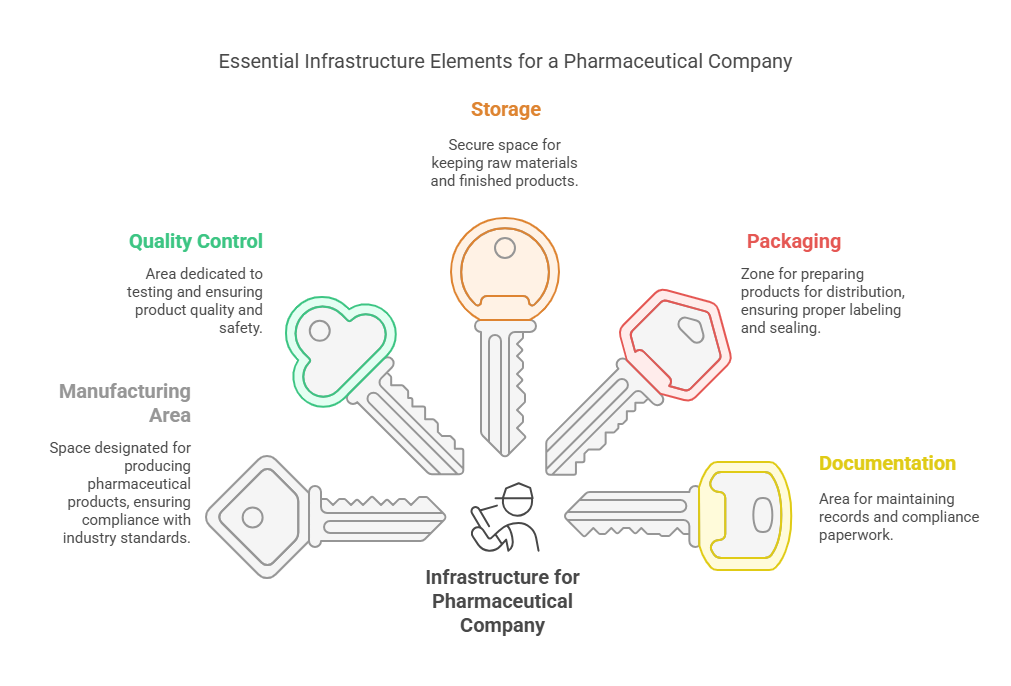
- Separate areas for:
- Manufacturing
- Quality Control
- Storage
- Packaging
- Documentation
- Temperature-controlled environments
- Clean room facilities
- Water treatment systems
Laboratory Setup
- Quality Control Lab
- Research & Development Facility
- Stability Testing Area
- Microbiological Testing Lab
Technical Expertise and Staffing
A critical aspect of how to start a pharmaceutical company in India involves obtaining the necessary licenses. Here’s what you need:
Required Technical Staff
Qualified Person (QP): A relevant expert working in the pharmaceutical or healthcare industry is responsible for placing products on the market only after confirming that the product fully meets regulatory standards and quality checks.
Quality Assurance (QA) Manager: Heads the systems and procedures that ensure products adhere to quality standards and regulatory requirements.
Production Manager: Responsible for the manufacturing operations, ensuring productivity, quality, and on-time delivery.
R&D Scientists: Responsible for researching and developing products to modify and create improved versions of existing products.
Quality Control (QC) Analysts: Analyze and evaluate raw materials and finished goods for conformity with predetermined quality criteria.
Regulatory Affairs Specialist: Makes sure the products are compliant with industry and governmental regulations, drafts documents, and deals with the filing for approvals.
Support Staff
Administrative Personnel: They manage the working of the office on a day-to-day basis such as managing communication, scheduling, filing documents and assisting the leader in achieving some of the organizations objectives.
Marketing Team: Create and implement campaigns to sell products or services, as well as promote them to the potential customers via particular mediums or channels.
Supply Chain Managers: Responsible for ordering, making, and transporting the goods in a smooth and organized manner from the supplier to the client.
Documentation Specialists: Prepare, maintain, and file relevant documents to ensure that the company meets the required legal, regulatory, and organizational requirements.
Financial Planning and Investment Analysis
Understanding the financial aspects is vital when learning how to start a pharmaceutical company in India: –
Location Strategy and Benefits
Initial Investment Breakdown
- Land and Building: ₹2-5 Crores
- Equipment and Machinery: ₹3-7 Crores
- Raw Materials: ₹1-2 Crores
- Licensing and Registration: ₹10-15 Lakhs
- Working Capital: ₹2-3 Crores
Funding Sources
- Bank Loans
- Government Schemes
- Venture Capital
- Private Equity
- Angel Investors
Choosing the right location is crucial when planning how to start a pharmaceutical company in India:
Prime Locations
- Maharashtra (Mumbai-Pune Belt)
- Gujarat (Ahmedabad-Vadodara Corridor)
- Telangana (Hyderabad)
- Himachal Pradesh
- Uttarakhand
Location Benefits
- Tax Incentives
- Infrastructure Support
- Skilled Workforce Availability
- Logistics Advantages
- Industry Clusters
Quality Management Systems
Quality is paramount when learning how to start a pharmaceutical company in India:
Quality Control Measures
Standard Operating Procedures (SOPs): A document prepared to provide detailed framework for processes to be followed to facilitate product quality and regulatory adherence.
Good Laboratory Practices (GLP): Principles that enable a laboratory study and its data to be reproducible and accurate.
Quality Assurance Protocols: Organized procedures that help verify a product’s quality and regulatory compliance.
Documentation Systems: The organized procedure by which all records concerning quality control processes are kept accurately and completely.
Batch Testing Procedures: Sampling and testing of production batches to ensure conformance to released quality criteria prior to batch release.
Compliance Requirements
- Regular Audits: Assessment of processes, systems and facilities at regular intervals to ensure conformance to the set regulatory and quality requirements.
- Quality Certifications: Compliance with and certification of recognized standards like ISO, GMP or approvals from FDA.
- Record Maintenance: Keeping records of all activities in a manner that is compliant with internal and external regulations.
- Staff Training Programs: Training provided at regular set intervals to enhance employees’ understanding of the quality standards, protocols, and compliance issues.
Product Portfolio Development
Strategic product selection is essential when planning how to start a pharmaceutical company in India:
Product Categories
- Generic Medicines
- Specialty Drugs
- OTC Products
- Nutraceuticals
- API Manufacturing
Market Entry Strategy
- Start with limited products
- Focus on high-demand segments
- Gradual portfolio expansion
- Market gap analysis
Distribution Network Establishment
Modifying a sturdy distribution network is the major thing to look into when you are learning how to initiate a pharmaceutical company in India:
Distribution Channels
- Wholesalers: Purchase pharmaceuticals in large volumes from providers and then sell them to retail stores, pharmacies, and health providers.
- Distributors: Help connect manufacturers with wholesalers for efficient distribution of products to various markets.
- Hospitals: Supply drugs and other related products for direct healthcare services such as hospital pharmacy use.
- Pharmacy Chains: Directly sell their products to over-the-counter retail pharmacy stores for sale to customers in specific areas.
- Government Tenders: Offer proposals to supply medicines to public health Institutions and medical programs run by the government.
Supply Chain Management
- Inventory Control: Use competent stock management systems to maintain adequate stock levels and avoid running out of stock or keeping excess stock.
- Cold Chain Management: Store and transport time and temperature-sensitive drugs such as vaccines and biological preparations under recommended conditions.
- Transportation Networks: Create effective supply chains for the distribution of products to all points of selling, including areas that are difficult to access.
- Warehouse Management: Maintain and use strategically positioned warehouses that are well equipped to receive and store bulk pharmaceutical products for onward distribution.
“You May Also Like This: – Top Benefits of Third Party Manufacturing Pharma,,
Regulatory Compliance and Documentation
Understanding regulatory requirements is vital for anyone wondering how to start a pharmaceutical company in India:
Key Regulations
- Drugs and Cosmetics Act
- DPCO (Drug Price Control Order)
- FDA Guidelines
- Environmental Laws
- Labor Laws
Documentation Requirements
- Batch Manufacturing Records
- Standard Testing Procedures
- Stability Data
- Validation Reports
- Quality Control Records
There are Two Most Important For Growing a Company
Marketing and Business Development
Effective marketing is crucial when learning how to start a pharmaceutical company in India:
Marketing Strategies
- Medical Representative Network
- Digital Marketing
- Trade Shows
- Scientific Symposiums
- Doctor Engagement Programs
Business Development
- Market Research
- Competition Analysis
- Product Positioning
- Pricing Strategy
- Brand Building
Risk Management and Challenges
Understanding potential risks is crucial when planning how to start a pharmaceutical company in India:
Common Challenges
- Regulatory Compliance
- Quality Maintenance
- Market Competition
- Price Controls
- Raw Material Costs
Risk Mitigation Strategies
- Insurance Coverage
- Quality Control Systems
- Legal Compliance
- Financial Planning
- Crisis Management
Conclusion
Initiating a pharmaceutical company in India demands thoughtful strategies, a considerable amount of money, and dedication towards compliance and quality. In case you are wondering how to start a pharmaceutical company in India, even though the process seems complex, proper planning and its execution can lead to magnificent rewards.
Achieving growth in the Indian market involves a deep understanding of the regulatory requirements, meeting quality standards, having a good set of team, and able to design good marketing strategies. It is indeed the right time to set foot in this sector considering that the Indian pharmaceutical market is always on the rise.
Do keep in mind that success in the pharmaceutical industry does not necessarily equate to profits: Public health has to come first, with the best safety and quality standards.
